YVOIRE
>
WHY YVOIRE


Distinguished HA raw materials
LG Chem has developed and manufactured hyaluronic acid pharmaceutical products 25 years.
1990HA R&D started with Producer Strain Development
1995Hyal 2000Ⓡ Inj.,1st HA Ophthalmic Viscoelastics launchedin Korea Hyruan plusⓇ Inj., 1st 3-Injection HA Osteoarticular
2000Viscosupplement launched in Korea
2010YVOIREⓇ, 1st Cross-linked HA Filler launched in Korea
2013YVOIREⓇ plus, 1st HA Filler with Lidocaine launched in Korea
-


-
Authorized quality
Reliable HA material listed to US
 DMF 1)
DMF 1)and certificated by
 DMF 2)
DMF 2)LG Chems’ non-animal origin HA has been marketed in various medical fields since 1995 with proven safety and efficacy. Its high molecular weight HA has been approved by the European Directorate for the Quality of Medicinesand its drug master file has been filed to the US FDA.
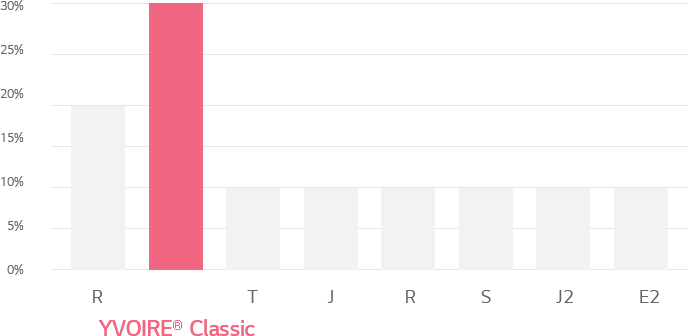
HICETM Cross-linking Technology
HICETM Cross-linking Technology, which enables to reach the maximum cross-linking ratio despite of “minimal use of cross-linkers” by dispersing the cross-linkers into highly concentrated HA during cross-linking reaction.
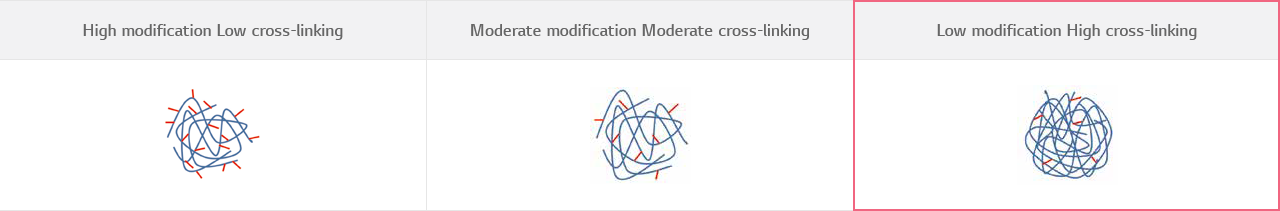
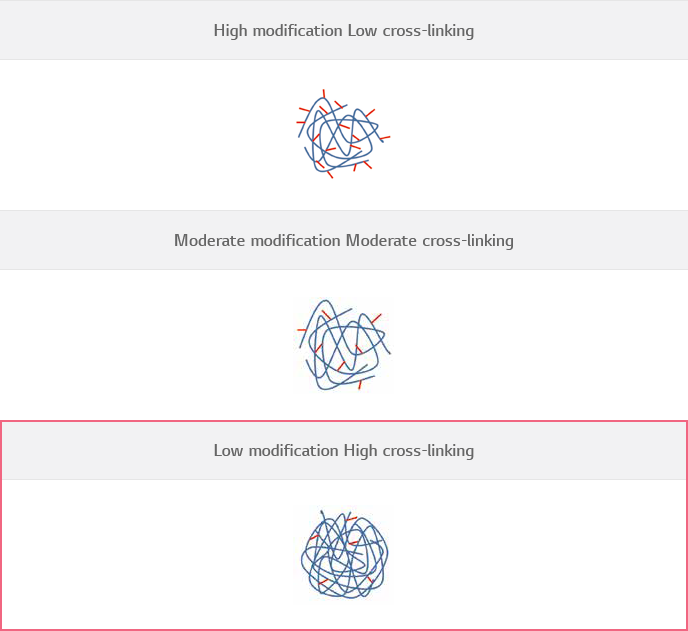
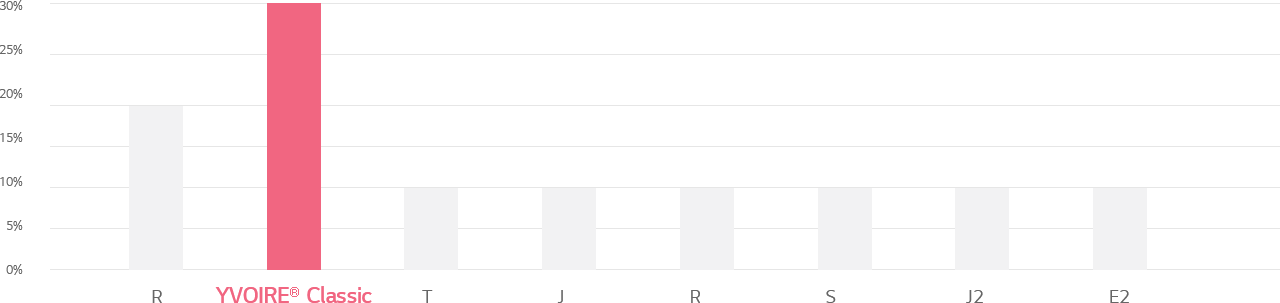
Cross-linking ratio of HA filler products
1. Drug master file No. 17416, FDA
2. Certification of Suitability of monographs of the European Pharmacopoeia, EDQM
3. Galderma’s patent, WO 2014/206701 A1